SAYPHA® FILLER w/ Lidocaine
$59.00
SAYPHA® FILLER w/ Lidocaine (by Croma) is a monophasic, cross-linked HA filler using S.M.A.R.T. technology. Designed for mid-dermis injection to correct moderate wrinkles and augment lips. Contains lidocaine. For professional use only where legally permitted.
Where can I buy this product?
SAYPHA® FILLER w/ Lidocaine may be available for purchase through Health Supplies Plus by licensed medical practitioners holding a valid medical license. First-time buyers can register an online account by providing required professional information, including medical license verification and a valid shipping address. Account verification is typically completed within 2 business hours, after which you can purchase medical supplies at competitive wholesale pricing. Registered members may also be eligible for volume discounts and receive dedicated customer service support.
Product Information
What is SAYPHA® FILLER w/ Lidocaine?
SAYPHA® FILLER w/ Lidocaine is a sterile, viscoelastic, injectable gel implant composed of cross-linked hyaluronic acid (HA) of non-animal origin, manufactured by Croma-Pharma GmbH (Austria). It is formulated using Croma’s proprietary S.M.A.R.T. (Supreme Monophasic and Reticulated Technology) process, described by the manufacturer as yielding a homogenous, monophasic gel.
This versatile formulation typically contains 23mg/mL of cross-linked hyaluronic acid and includes 0.3% lidocaine hydrochloride to enhance patient comfort during the injection procedure. SAYPHA® FILLER is designed for injection into the mid-to-deep dermis to correct moderate to severe facial wrinkles and folds, and for lip augmentation.
Storage: Store according to manufacturer guidelines, typically between 2°C and 25°C (36°F and 77°F). Do not freeze. Protect from light and moisture.
Authenticity: Health Supplies Plus guarantees that SAYPHA® products sourced are authentic from the manufacturer or authorized distributors.
Note: For professional use only by trained and licensed practitioners in jurisdictions where its use is legally permitted.
How does SAYPHA® FILLER w/ Lidocaine work?
SAYPHA® FILLER w/ Lidocaine utilizes the S.M.A.R.T. technology to create a monophasic, cross-linked HA gel intended for versatile application. When injected into the mid-to-deep dermis, it adds volume to physically lift and smooth moderate to severe facial wrinkles and folds, such as nasolabial folds, marionette lines, and forehead furrows.
Its properties are also designed for lip enhancement, allowing for volume augmentation and contouring. The manufacturer claims the S.M.A.R.T. process ensures smooth integration and natural-feeling results. The hydrophilic nature of HA contributes to tissue hydration.
Treatment results are intended to be visible immediately. The manufacturer suggests a duration of effect lasting approximately 6 to 9 months, although individual results will vary based on patient factors, injection site, volume, and technique. The integrated lidocaine helps manage patient comfort during the procedure.
Indications for Use
Based on manufacturer information and common use outside the US, SAYPHA® FILLER w/ Lidocaine is typically indicated for adults seeking correction of moderate facial lines and lip enhancement:
- Correction of moderate to severe wrinkles and folds (e.g., nasolabial folds, marionette lines, forehead furrows).
- Lip augmentation for volume and contouring.
- Smoothing perioral lines.
Appropriate patient selection and correct injection depth (mid-to-deep dermis or lip mucosa) are crucial where its use is permitted.
Primary Benefits
- Versatile filler designed for moderate wrinkles, folds, and lip augmentation.
- Utilizes S.M.A.R.T. technology for a monophasic gel structure.
- Provides immediate volumizing and smoothing results.
- Results claimed to last 6-9 months.
- Contains lidocaine for improved patient comfort during injection.
Side Effects and Safety Information
Common side effects expected with HA filler injections include temporary, injection-site related reactions:
- Swelling
- Bruising
- Redness
- Tenderness or Pain
- Itching
- Firmness or Lumps/Bumps
- Inflammation
Severe Adverse Events:
As with any dermal filler injection, there are risks of more serious complications. Practitioners must be prepared to manage adverse events. Potential severe adverse events include:
- Vascular Occlusion (VO): Accidental injection into a blood vessel can obstruct blood flow, potentially leading to tissue necrosis (tissue death), scarring, or (in rare cases involving facial arteries, especially labial or angular) blindness or stroke. Prompt recognition and management protocols are crucial.
- Severe allergic reactions or hypersensitivity to HA or lidocaine
- Inflammation, infection, or abscess formation
- Granuloma or persistent nodule formation
- Migration of filler
- Prolonged pain or numbness
- Herpetic eruptions (cold sores) in susceptible individuals
- Hematomas
Any signs of vascular compromise, infection, hypersensitivity, or persistent adverse reactions require immediate medical evaluation.
| Manufacturer | Croma |
|---|---|
| Active Substance(s) | Sodium hyaluronate, lidocaine |
| Strength | 23mg/ml, 3mg/ml |
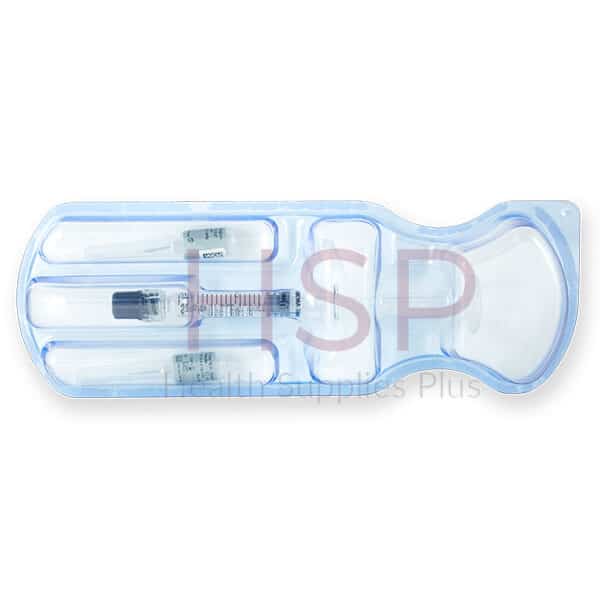
| Pack Size | 1-1ml prefilled syringe |
| Accessories | Package insert, 2-27G 1/2” needles, 2 traceability labels |